During the last few decades we have observed how the discoveries of science applied to medicine have been instrumental in increasing human life span. Moreover, the objective of scientists is to extend human health span along with life span. In other words, more individuals should be able to reach the age of 122 years (nowadays) and should be healthy during longer periods of time. Let´s Imagine feeling the strength and fitness proper of 40 years at the age of 80 or 90. If this were the case, why is the female fertility peak restricted from late teens to late twenties? This question is not only raised for the purpose of deciding the best age for childbearing, but also because the risk of suffering from aging-associated diseases is increased when ovaries are aged.
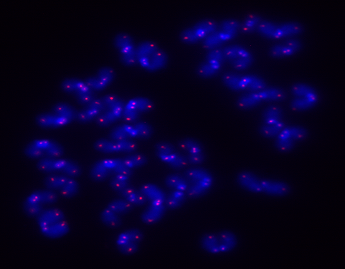
Several pathways are implicated in longevity1, but the one in charge of chromosome protection (called the telomere pathway) is a critical one because its failure disturbs and alters other pathways which are also involved in aging1. Telomeres are special structures localized at the ends of chromosomes which protect them from degradation or fusions2, and therefore, protects the cells from genome instability, which is a hallmark of cancer and aging. Telomeres consist of tandem repeats of the DNA sequence “TTAGGG” in mammals, reaching a length of 10 to 15kb in humans and 40kb in mice. The telomeric DNA sequence is wrapped around nucleosomes (a group of proteins specialized in maintaining chromosome structure) which are marked to keep telomeres transcriptionally controlled. Telomeres are bound by a complex of six proteins called Shelterin to ensure protection. Another layer of protection is safeguarded by long-non-coding RNA molecules called TERRAS which are attracted and bound to telomeres3,4, 5.
Telomeres cannot be fully duplicated by the replication machinery of cells; consequently, when cells divide, telomere length is reduced. If telomeres reach a critically short length, the DNA becomes unprotected3,4,5. Then, telomeres are detected as double strand breaks and cells activate DNA repair pathways, which lead to cell senescence or apoptosis. This means that these cells will not be able to divide anymore. If senescent cells are increased in our body, the regenerative capacity of tissues and organs is reduced and the organism ages6. Telomerase is an enzyme that can add repeats to chromosome ends to counteract telomere shortening during replication, therefore preventing telomeres from reaching a critically short length7. However, telomerase activity is not detectable in most cells of the organism, but is found in embryonic and adult stem cells as well as cancer cells. The lack of telomerase activity in most cells of the organism protects them from becoming cancerous, since most cancer cells reactivate telomerase8.
Organismal aging due to telomere attrition have been shown in telomerase-deficient mice which accumulate short telomeres and age very fast but are cancer resistant9. Also, humans with mutations in telomerase age faster and suffer from severe diseases related to poor regenerative capacity, such as dyskeratosis congenita10.
Several lines of evidence support the notion that telomere pathway is involved in fertility. In mice lacking telomerase, they accumulate short telomeres and fertility is lost by the sixth generation as opposed to control mice that are fertile for many generations. Both female and male reproductive organs and germ cell production are deteriorated when telomerase is not present11,12. Regarding the female reproductive system, a low production of oocytes together with poor embryo development and defects in uterine structure/function are observed. In addition, in oocytes of the fourth generation lacking telomerase, oocyte structure is disrupted. Chromosomes are misaligned on the metaphase plate and the mitotic spindle is aberrant, suggesting that telomeres may have a role in MII oocyte organization. Regarding male reproduction, a reduction in spermatocyte production was observed11,12.
Recent evidence shows that the telomere pathway is implicated in human infertility cases. Shorter telomeres and low or undetectable telomerase activity has been found in peripheral blood leukocytes and granulosa cells (GC; cells which nurture and support maturation of oocytes) of women with ovarian insufficiency and ovarian failure13,14. In men, accumulation of sperm with short and dysfunctional telomeres have been found in cases of subfertility and short telomeres seem to be more abundant in oligospermic samples15, 16.
Despite the efforts to understand the implication of telomere biology in fertility, more work is needed to fully understand the mechanisms that orchestrate infertility and aging, to attempt to
extend the period of safe reproductive life, to ensure better health span at older ages as well as to open a broader window for childbearing decision.
Post by Elisa Varela
Bibliography
- López-Otín, C., Bkasco, M.A., Partridge, L., Derrano, M. and Kroemer, G. The hallmarks of aging. 2013. Cell 153: 1194 – 1217.
- Blackburn, E., Epel, E.S. and Lin, J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. 2015. Science 350: 1193 – 1198.
- de Lange, T. Shelterin the protein complex that shapes and safeguards human telomeres. 2005. Genes Dev. 19: 2100 – 2110.
- de Lange, T. Shelterin-mediated telomere protection. 2018. Annu. Rev. Genet 52: 223 – 247.
- Martínez, P. and Blasco, M.A. Role of shelterins in cancer and aging. 2010. Aging Cell 9: 653 – 663.
- Donate, L.E. and Blasco M.A. Telomeres in cancer and ageing. 2011. Philos. Trans. R. Soc. Lon. B. Biol. Sci. 366: 76 – 84.
- Greider, C.W. and Blackburn, E.H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. 1985. Cell 43: 405 – 413.
- Maciejowski, J. and de Lange, T. Telomeres in cacner: tumour suppression and genome instability. 2017. Nature Reviews 18: 175 – 186.
- Blasco, M.A., Lee, H., Hande, M.P. Samper, E. Lansdorp, P.M. DePinho, R.A. and Greider, C. W. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91: 25 – 34.
- Merck, S. J. and Armanios, M. Shall we call them “telomere-mediated”? Renaming the idiopathic after the cause is found. 2016. Eur. Respir 48: 1556 – 1558.
- Lee, H., Blasco, M.A., Gottlieb. G.J., Horner, J.W., Greider, C.W. and DePinho, R.A. Nature 1998. Nature 392: 569 – 574.
- Liu, L. Blasco, M.A. and Keefe, D. Requirement of functional telomeres for metaphase chromosome alignments and integrity of meiotic spindles. 2002 EMBO Reports 31: 230 – 234.
- Xu, X., Chen, X., Zhang, X., Liu, Y., Wang, Z., Wang, P., Du, Y., Qin, Y. and Chen, Z. 2017. Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Human Reproduction 32: 201 – 207.
- Butts, S., Riethman, H., Ratcliffe, S., Shaunik, A., Coutifairs, C. and Barnhart, K. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. 2009. J. Clin. Endocrinol. Metab. 94: 4835 – 4843.
- Cariati, F., Jaroudi, S., Alfarawati, S. Raberi, A. Alviggi, C., Pivonello, R. and Wells, D. Investigation of sperm telomere length as a potential marker of paternal genome integrity and semen quality. 2016. Reproductive Biomedicine online 33: 404 – 411.
- Biron-Shental, T., Wiser, A., Hershko-Klement, A., Markovitch, O., Amiel, A. and Berkovitch, A. Sub-fertile sperm cells exemplify telomere dysfunction. 2018. J. Assist Reprod. Genet. 35: 143 – 148.