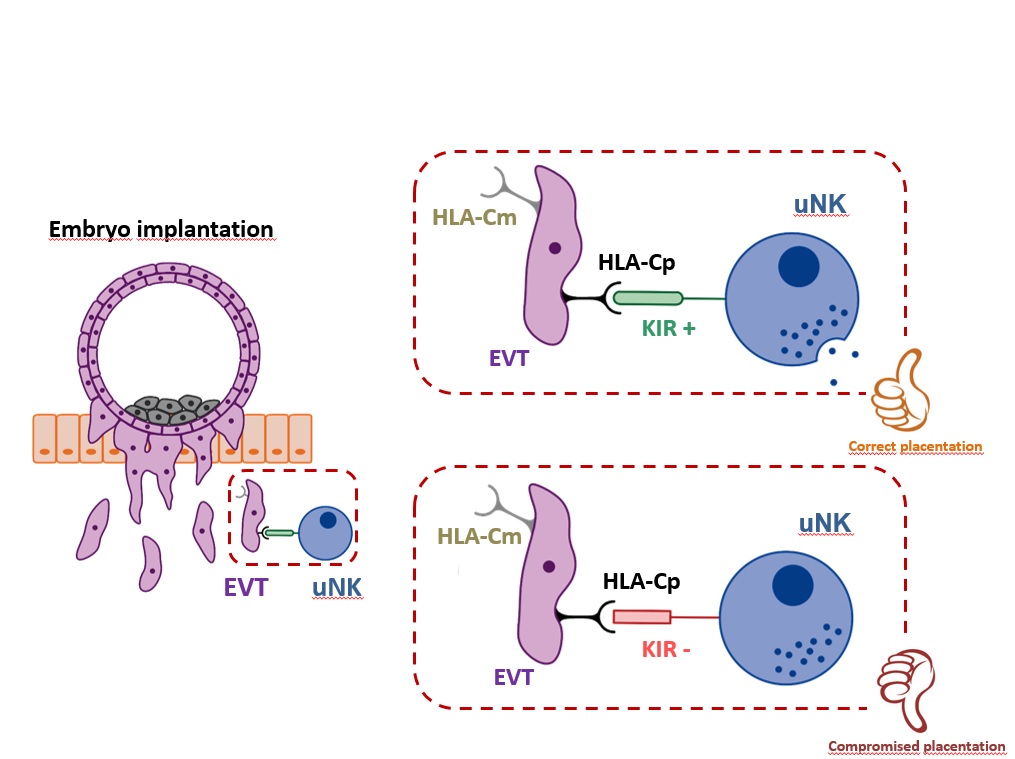
In terms of maternal-fetal allorecognition, interactions between members of the Killer Immunoglobulin-like Receptor (KIR) family expressed by uNK cells binding to trophoblast Human Leukocyte Antigen-C (HLA-C) molecules are of particular interest, as both maternal KIR and fetal HLA-C genes are highly polymorphic. There will be different maternal/fetal genetic combinations in each pregnancy. Placentation is regulated by interactions between maternal KIRs, and fetal HLA-C. Both maternal and paternal HLA-C are presented to KIRs. Insufficient invasion of the uterine lining by trophoblasts and vascular conversion in the decidua are thought to be the primary defect in disorders such as recurrent miscarriages (RM), preeclampsia, and fetal growth restriction (FGR).
Several studies conducted in natural pregnancies by Hiby SE and Ashley Moffett showed that women who have a KIR AA genotype (two KIR A haplotypes) are at risk of pre-eclampsia and other pregnancy disorders when the fetus has more HLA-C2 genes than the mother and when additional fetal HLA-C2 alleles are of paternal origin. Thus, depending on the particular KIR–HLA-C interaction, the trophoblast cell invasion is regulated.
In ART pregnancies where donor oocytes or embryos are used, the embryo shares no maternal ‘self’ genes, as both sets of chromosomes are derived from ‘non-self’ individuals. In other words, the oocyte HLA-C allele is genetically different from the surrogate mother’s HLA-C alleles, and thus the oocyte HLA-C allele represents an additional “paternal” or non-self HLA-C, increasing the number of foreign HLA-C alleles to be faced by the mother immune uterine cells.
Previous research has highlighted the risk for women and their babies in oocyte donation pregnancies. Consistent findings are the increased risk of pregnancy-induced hypertension and other Great Obstetrical Syndromes (GOS) (preeclampsia and FGR), even when controlling for age and singleton pregnancies.
For the first time in 2014, our group reported a significantly decreased live birth rate (LBR) after a DET with donated oocytes in KIR AA patients when compared to KIR AB and KIR BB.
A prospective observational study performed in IVIRMA Clinics including patients with an unknown etiology of their recurrent embryo losses undergoing to IVF and egg donation observed a higher miscarriage rate after DETs in KIR AA mothers (47·8% after egg donation and 37·5% after IVF) compared to those with KIR AB (10·5% after egg donation and 12·5% after IVF) or KIR BB (6·7% and 0% after egg donation or IVF, respectively). A significantly decreased LBR was also observed after DETs in oocyte donation cycles when the patient was KIR AA (4·3%) compared to KIR AB (26·3%) or BB (46·7%) (p=0·009).
The study observed a significant decrease in the LBR for KIR AA patients when embryos who had more HLA-C2 genes than the mother were transferred when compared to those with fewer HLA-C2 genes than the mother (28·3 vs 57·1%, p<0·02).
The miscarriage rate increased as the embryo HLA-C2 load increased in KIR AA patients. This trend was not observed in KIR AB or BB patients. Similarly, no differences were observed in the LBR based on the embryo HLA-C2 load in KIR AB or BB patients.
Implications of all the available evidence
The oocyte donation is an excellent model to study the effects of maternal KIR/fetal HLA-C interactions. The HLA-C2 extra load could overtax maternal-fetal tolerance, with a negative impact on placentation and an increase risk for RM and pre-eclampsia.
There is previous evidence that human reproductive success depends to some extent upon KIR and HLA-C genes as these are be responsible for maintaining balanced polymorphisms between the HLA-C1 and HLA-C2 groups and the A and B KIR haplotypes. However, this natural human evolution is not currently being taken into consideration during ART, and our findings show that maternal KIR genotype and fetal HLA-C have an impact on the LBR after IVF cycles, especially with DETs and even more so in egg donation.
SETs improve the reproductive outcome, although the mother’s uterine genetic background remains impactful. There is a negative impact on the LBR when there is increased presence of fetal HLA-C2 in KIR AA women. The selection of HLA-C1 over HLA-C2 donors, combined with SETs, could have a positive impact on the LBR in KIR AA patients.
Author: Diana Alecsandru, M.D., Ph.D.