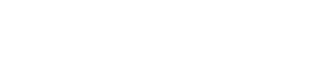All – care providers and patients alike – understand that Assisted Reproductive Techiniques (ART) do not succeed every time because of technical imperfections and limitations of human reproduction.
When facing repeated ART failures, clinicians should try to determine whether this is caused by a problem residing in the embryo or endometrium.
Easier said than done.
The debate over the definition of Recurrent implantation failure (RIF) has increased with stricter parameters utilized as treatment outcomes have improved. Despite numerous publications regarding RIF, it remains one of the most enigmatic areas of ART with no universal definition. Thus far, no medical society has assumed the challenge of suggesting a definition for RIF or indicating which measures need to be undertaken in case of repeated ART failures.
The parameter most often used for defining RIF has been the number of transferred embryos with failed outcome. Early investigators proposed that RIF be defined as ART failure after at least 10 embryos transferred. More recently, reflecting the progress made in reproductive biology, more conservative definitions have been proposed. RIF is indeed declared to exist after at least four unsuccessful transfers of good quality embryos.(1)
For obvious reasons, IVF cycle failures can result from one or multiple factors, and not necessarily the same factor(s) each time.
Many women with repetitive ART failures have chromosomally abnormal, or aneuploid, embryos. Today, however, the principal genetic error affecting embryos – whole chromosome aneuploidy – can be accounted for by performing PGT-A. Hence, when PGT-A is performed genetically abnormal embryos are not transferred and thus, no longer part of the RIF population. Although implantation rates of euploid embryos are markedly improved as compared to non-tested embryos, not all euploid embryos implant. Maternal age has an effect; however small, on implantation rates of euploid embryos as early as age 33 years, according to recent reports by IVIRMA group. (2)
With the advent of the genic era, efforts have been deployed for defining endometrial changes linked to the window of implantation (WOI) according to the expression of several key genes in the endometrium. Notably, researchers proposed the endometrial receptivity analysis (ERA) test for assessing the quality of endometrial receptivity and advancement of endometrial changes.(3) Other receptivity tests have been proposed notably, based on the expression of 11 genes selected for their role in the control of endometrial receptivity or miRNA profiles.(4) While of the utmost interest, these approaches have not yet been validated in formal RCTs. Reviewing the assessment of endometrial receptivity, other research underscores the role of BCL6 expression in the endometrium and progesterone resistance in women with endometriosis as predictors of poor implantation(5).The recent report of unaltered implantation rates following frozen euploid blastocyst transfers in endometriosis indicates that these detrimental effects of endometriosis appear to be neutralized in E2 and progesterone cycles used for FETs. Another strategy was pursued by a French group who tried to determine an ideal immunologic endometrial profile, which would optimize implantation rates notably, in RIF. (6)There too, the theory is interesting, but the approach has not been validated in a formal RCT
In light of the above, we felt that failing at least three consecutive single euploid frozen blastocyst transfers in women with morphological normal uteri would reasonably define RIF.
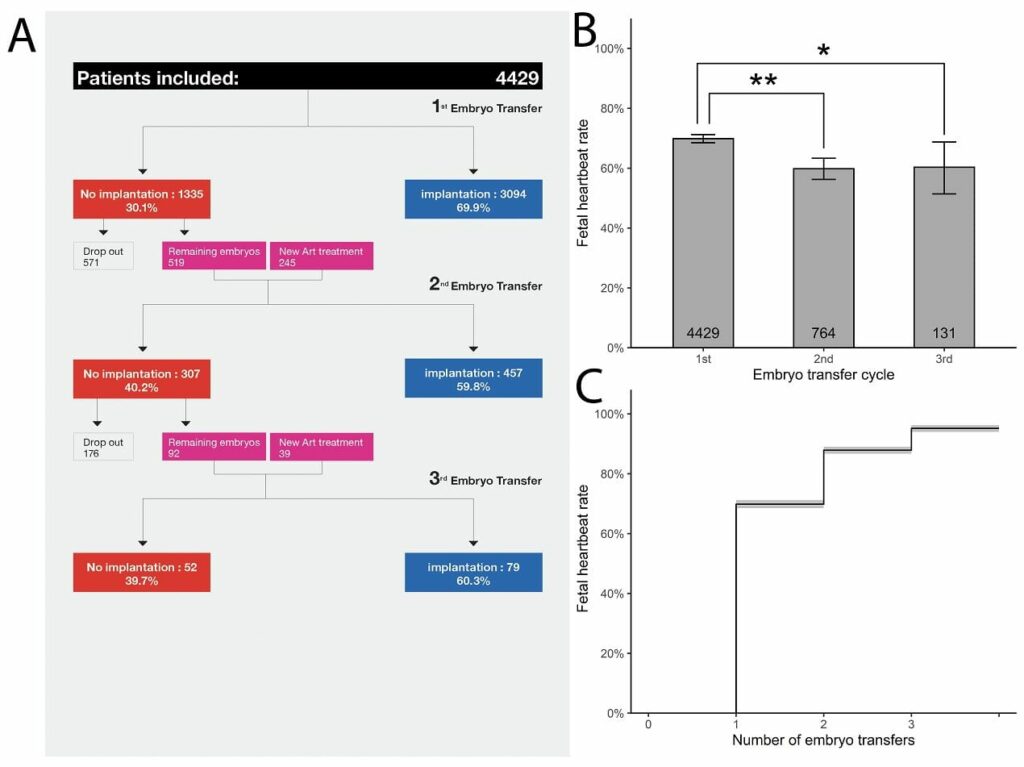
This is the reason why we conducted a large retrospective study on all patients (in total 4429 patients) with up to three consecutive single euploid frozen embryo transfers between January 2012 and July 2018 in IVIRMA New Jersey. Our findings suggest that true RIF is rare. For those patients with the ability to make euploid blastocysts, 95.2% would achieve clinical pregnancy with 3 embryos transferred. (Fig 1)
As evidenced by this study, the implantation rates decline minimally with increasing transfers but the fact that they remain high suggests that 5% who fail to implant after three attempts may largely be victim of simple probabilities/statistics and that additional transfers offer hope of good outcome. Our result indicate that the possible endometrial causes of RIF are very rare thereby throwing into doubts all endometrial receptivity assay, none of which shown merit in RCTs.
In conclusion, the endometrial receptivity assays available today may not be helpful for handling women with RIF with a morphologically normal uterus.
It may be reasonable to simply have the patient undergo another frozen embryo transfer. The tendency for both patient and clinicians is to want to change something in the face of failure.
These data suggest that simply staying the course yields a very high chance of success.
- Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T et al. Recurrent implantation failure: definition and management. Reprod Biomed Online 2014;28:14-38.
- Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril 2014;101:656-63.e1.
- Bassil R, Casper R, Samara N, Hsieh TB, Barzilay E, Orvieto R et al. Does the endometrial receptivity array really provide personalized embryo transfer? J Assist Reprod Genet 2018;35:1301-5.
- Neves AR, Devesa M, Martínez F, Garcia-Martinez S, Rodriguez I, Polyzos NP et al. What is the clinical impact of the endometrial receptivity array in PGT-A and oocyte donation cycles? J Assist Reprod Genet 2019;36:1901-8.
- Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril 2019;111:611-7.
- Lédée N, Petitbarat M, Prat-Ellenberg L, Dray G, Cassuto GN, Chevrier L et al. Endometrial Immune Profiling: A Method to Design Personalized Care in Assisted Reproductive Medicine. Frontiers in immunology 2020;11:1032.